2.6.5.8 Ester Volatile Oils
The ‘Ester Volatile Oils’ essentially attribute their flavouring characteristics, odour, aroma and specific perfume by virtue of the presence of a good number of naturally occurring esters, most common among which are the acetates of borneol, geraniol and terpineol. However, it is an age-old practice to allow the maturation or ageing of such ester-containing perfumes in order to enhance the process of esterification in situ thereby ultimately improving the overall aroma and bouquet of the volatile oil. Incidentally, there are certain exceptions, such as: the ‘Oil of Wintergreen’ which contains upto 99% of methyl salicylate (an ester). Classification The ester volatile oils may be classified conveniently into three categories as follows:
(i) Esters of Aliphatic Acids,
(ii) Esters of Aromatic Acids, and
(iii) Esters containing Nitrogen.
These different categories of ester volatile oils
shall be discussed along with their typical examples as under:
2.6.5.8.1 Esters of Aliphatic Acids The
typical examples of esters of the aliphatic acids are, namely: Geranyl
acetate, Linalyl acetate.
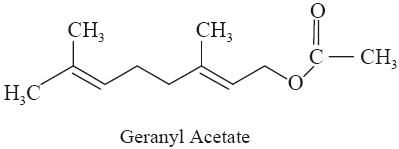
A. Geranyl Acetate
Chemical Structure 3, 7-Dimethyl-2,
6-octadien-8-acetate; (C12H20O2). It is an
olefenic terpene acetate mostly found in a number of essential oils.
Occurrence The ester is very widely
distributed in a variety of essential oils, such as: oil of citronella,
petit grain, lemon-grass, coriander, lavender etc. It is also found in Satureja
montana L. (Lamiaceae)-Winter Savory, White Thyme, Spanish
Savory; and Tilia europaea L. (Tiliaceae)- Lime Tree (Europe),
Linden Tree (America).
Isolation It may be obtained from the rich
source of volatile oil containing greanyl acetate by fractional
distillation under vacuum.
Characteristic Features It is a colourless
liquid having a pleasant aromatic fragrance resembling to that of rose. It
boils at 242-245°C with decomposition at atmospheric pressure.
Identification The ester on saponification
with alcoholic KOH yields geraniol and acetic acid as the products of reaction.
The former may be identified by examining its physical parameters, for instance:
bp757 229-230°C; bp12 114-115°C; d204
0.8894; n20D 1.4766; uvmax 190-195 nm (ε 18000).
Uses
1. It is used abundantly in perfumery.
2. It is also employed in making cosmetics and
various types of toilet soaps.
B. Linalyl Acetate
Synonym Bergamot.
Chemical Structure 3, 7-Dimethyl-1,
6-octadien-3-yl acetate; (C12H20O2). It is
also an olefinic terpene acetate and regarded as the most valuable constituent
of bergamot and lavender oils.
Occurrence It is found in a number of volatile
oils, namely: Lavandula angustifolia Mill.
(Lamiaceae)-Lavender, True or Common
Lavender; Salvia selarea L. (Lamiaceae)-Clary, Cleareye,
Muscatel Sage; Satureja montana L. (Lamiaceae)-Winter
Savory, White Thyme, Spanish Savory; Thymus vulgaris L. (Lamiaceae)-Common
Thyme; Tilia europaea L. (Tiliaceae)-Linden Tree (America),
Lime Tree (Europe).
Isolation It may be obtained from lavender
oil or bergamot oil by subjecting it to distillation under very high
vacuum, because on distillation at atmospheric pressure or with steam
distillation linalyl acetate gets hydrolysed rapidly and decomposed eventually.
Characteristic Features It is a colourless
oily liquid having a very pleasant fruity odour of bergamot oil. Its
physical properties are: d204 0.885; bp 220°C; n20D
1.4460. It is almost insoluble inwater but miscible freely with ether and
alcohol.
Identification Linalyl acetate upon
saponification with alcoholic KOH yields linalool (linalol) and
acetic acid. The dl-form of linalool has a bp720 194-197°C.
Uses It is used extensively in perfumery.
2.6.5.8.2 Esters of Aromatic Acids The
various esters that are associated with aromatic acids and found in volatile
oils are: Benzyl benzoate, Cinnamyl Cinnamate; Methyl Salicylate.
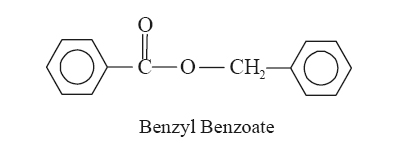
A. Benzyl Benzoate
Synonyms Ascabin; Venzonate; Ascabiol.
Chemical Structure Benzoic acid phenyl methyl
ester; (C14H12O2).
Occurrence It is contained in Peru and Tolu
balsams. It is also found in a variety of volatile oils, such as: Cananga
odorata (Lam.) Hook. f. & Thomas (Annonaceae)-Ylang-Ylang,
Cananga; Cinnamonum verum J S Presl (Lauraceae)-Ceylon
Cinnamon; Myroxylon balsamum Var. Pereirae (Royle) Harms. (Fabaceae)-Balsam
of Peru; Peumus boldus Molina (Monimiaceae)-Baldo; Vanilla
planifolia Andr. (Orchidaceae)-Vanilla.
Isolation Benzyl benzoate may be isolated by
cooling the corresponding fraction to a very low temperature when it gets separated
as a solid (mp 21°C). It may also be further recrystallized from chloroform or
ether.
Characteristic Features It is an oily liquid
or leaflets, having a faint, pleasant aromatic odour. It possesses a sharp
burning taste. Its physical characteristics are: mp 21°C; d204
1.118; bp 323-324°C; bp16 189-191°C; bp4.5 156°C, and n21D
1.5681. It is sparingly volatile with steam. It is insoluble in water or
glycerol, but miscible with alcohol, chloroform, ether and oils.
Identification The benzyl benzoate on saponification
yields the two products of reaction i.e., benzoic acid and benzyl
alcohol that may be identified by carrying out specific tests for these compounds.
Uses
1. It is used extensively as a diluent and solvent of
solid aromatics e.g., artificial musk.
2. By virtue of its low volatility benzyl benzoate is
employed as a fixative in perfume composition.
3. It is used as a solvent for cellulose acetate and
nitrocellulose.
4. It serves as a substitute for camphor in celluloid
and plastic pyroxylin compounds.
5. It is also employed in confectionery and chewing
gum flavours.
B. Cinnamyl Cinnamate
Synonyms Cinnyl cinnamate; Styracin.
Chemical Structure 3-Phenyl-2-propenoic acid
3-phenyl-2-propenyl ester.
Occurrence It occurs in the buds of Populus
balsamifer L., (Family: Salicaceae)-Goris; Lavanga scandens Buch-Ham.,
Lavangalata-Baslas; and Styrox Benzoin Dryander (Family: Styracaceae)-Benzoin,
Sumatra Benzoin, Styrax.
Isolation It may be obtained from the volatile
oil fraction by chilling it to low temperature and collecting the solids having
mp 44°C.
Characteristic Features The characteristic
features of the trans-trans-cinnamyl cinnamate are: mp 44°C;
uvmax (95% ethanol): 216, 223 nm (log ε 3.45, 3.25). It is practically
insoluble in water but sparingly soluble in cold ethanol and soluble in ether
(1 g in 3 ml).
Identification On saponification with
alcoholic KOH this ester gives rise to cinnamic acid and cinnamyl alcohol which
may be further identified by performing their specific tests.
Uses
1. It is used in perfumery.
2. It is also employed in making toilet soaps etc.
C. Methyl Salicylate
Synonyms Winter green oil; Betula oil; Sweet birch
oil; Teabery oil.
Chemical Structure 2-Hydroxybenzoic acid methyl
ester; (C8H8O3)
Occurrence It is largely found in a variety of
medicinal plants, namely: Flowers of Acacia farnesiana (L.) Willd.
(Family: Fabaceae)-Cassie, Huisache; Cananga odorata (Lam.)
Hook. f. & Thoms (Family: Annonaceae)-Cananga,
Ylang-Ylang; Leaves of Chenopodium ambrosioides L. (Family: Chenopodiaceae)-Wormseed;
Erythroxylum coca Lam. (Family: Erythroxylaceae)-Coca;
Flowerbuds of Filipendula ulmaria (L.) Maxim (Family: Rosaceae)-Meadowsweet,
Queen of the Meadow; Twigs of Gaultheria procumbens L.
(Family: Ericaceae)-Wintergreen, Teaberry, Boxberry; Bark of Betula
lenta L. (Family: Betulaceae)-Sweet Birch. However, oil of
wintergreen contains upto 99% methyl salicylate. It is pertinent to
mention here that in several aromatic medicinal plants, for instance: Wintergreen,
the active chemical constituent i.e., methyl salicylate does
not occur as such, but is present in the form of a glucoside known
as Gaultherin which upon enzymatic hydrolysis gives methyl salicylate
and primeverose (glucoxylose) as shown under:
Isolation Methyl salicylate may be obtained
from gaultherin by enzymatic hydrolysis and then subjecting the resulting
products of reaction to very low temperature when the former gets solidified at
– 8.6°C and hence may be separated easily.
Characteristic Features It is a colourless,
yellowish or reddish, oily liquid. Its odour and taste resembles to that of
gaultheria. Its physical parameters are: mp – 8.6°C; bp 220-224°C; d2525
1.184; d of the natural ester is ~ 1.180; and n20D
1.535-1.538. It is very sparingly soluble in water (1 g in 1500 ml), but freely
soluble in chloroform and ether. It is, however, miscible with alcohol and glacial
acetic acid.
Identifications
1. It develops a red-violet colouration on being
treated with cold saturated aqueous solution of FeCl3, that lasts
for about 15 minutes.
2. Methyl salicylate readily forms a soluble
ester-salt with a moderately concentrated aqueous solution of KOH as potassium
methyl salicylate.
3. Upon saponification the ester yields salicylic
acid (mp 158°C) and methanol respectively.
4. Methyl salicylate may also be identified by
the formation of several derivatives as stated below:
(i) Methylo-acetoxy benzoate (mp
52-52.5°C)—with Acetic Anhydride,
(ii) Methyl-o-benzoxy benzoate (mp 92°C)—with
Benzoyl Chloride,
(iii) o-Cabomethoxyphenyl-N-phenyl
urethane—with Phenylisocyanate.
Uses
1. Methyl salicylate has local irritant,
antirheumatic and antiseptic properties. It is an important ingredient of Iodex(R)
ointment for relief of pain in several conditions like, pulled muscle,
muscular pain, pain in joints, etc.
2. It also finds its extensive usage in a variety of
products, such as: flavouring of food products, beverages, candies,
confectionery, toothpastes, mouth washes, gargles, and pharmaceutical
preparations.
3. It is also used in perfumery.
2.6.5.8.3 Esters Containing Nitrogen The
specific example of an ester containing nitrogen is
Methyl Anthranilate which is present in
several volatile oils. It is described below:
A. Methyl Anthranilate
Synonyms Neroli Oil (Artificial).
Chemical Structure 2-Aminobenzoic acid methyl
ester, (C8H9NO2).
17 ml of distilled water. The resulting methyl
anthranilate sulphate thus obtained gets crystallized in the cold, which may be
further purified by recrystallization from alcohol. Finally the pure desired ester
is regenerated by treatment with dilute NaOH solution (2 N) carefully.
Characteristic Features It is a crystalline
mass having a powerful pleasant taste. It has a peculiar odour that mostly
resembles to orange blossoms and certain varieties of grape. It gives an inherent
blue-violet fluorescence which is distinctly visible in any volatile oil
containing it. Methyl anthranilate has the following physical
parameters, namely: d 1.168; mp 24-25°C; bp15 135.5°C.
It is slightly soluble in water, but freely soluble
in ethanol and ether.
Identification It may be identified by
preparing its derivatives, such as: Picrate (mp 104°C); Benzoate (mp 100°C).
Uses
1. It is used frequently as a perfume for ointments.
2. It is also employed for the manufacture of
synthetic perfumes.
Occurrence It occurs in a good number of
medicinal herbs, for instance: Flowers of Robinia pseudoacacia L.
(Family: Fabaceae)-Black Locust, False Acacia; Citrus sinensis
(Linn.) Osbeck. (Family: Rutaceae)-Sweet Orange; Cananga
odorata (Lam.) Hook. f. & Thoms. (Family:
Annonaceae)-Ylang-Ylang, Cananga; Jasminum
officinale Linn. var grandiflorum Bailey., (Family: Oleaceae)-Jasmine.
It is also found in bergamot, other essential oils and in grape juice. It
is also obtained synthetically by carrying out the esterification of
anthranilic acid with methanol in the presence of HCl.
Isolation Methyl anthranilate may be isolated
from the essential oils very conveniently by shaking the volatile oil with cold
dilute sulphuic acid (2 N) i.e., 1 ml of conc. H2SO4
dissolved slowly in
17 ml of distilled water. The resulting methyl
anthranilate sulphate thus obtained gets crystallized in the cold, which may be
further purified by recrystallization from alcohol. Finally the pure desired ester
is regenerated by treatment with dilute NaOH solution (2 N) carefully.
Characteristic Features It is a crystalline
mass having a powerful pleasant taste. It has a peculiar odour that mostly
resembles to orange blossoms and certain varieties of grape. It gives an
inherent blue-violet fluorescence which is distinctly visible in any volatile
oil containing it. Methyl anthranilate has the following physical
parameters, namely: d 1.168; mp 24-25°C; bp15 135.5°C.
It is slightly soluble in water, but freely soluble
in ethanol and ether.
Identification It may be identified by
preparing its derivatives, such as: Picrate (mp 104°C); Benzoate (mp 100°C).
Uses
1. It is used frequently as a perfume for ointments.
2. It is also employed for the manufacture of
synthetic perfumes.
Source:Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar
Source:Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar







1 Comment:
I was worried about my dull hairs and I found Ester Volatile Oils as a rescue. Thanks to your website for sharing such valuable information with us.
Regards
certified organic essential oils are derived from plants
Post a Comment