2.1 Monoterpenoids
In general,
monoterpenoids represent a structurally diverse class of compounds may
be categorised into nearly 35 varying structural analogues. However, the most
commonly occurring structural variations are of
the following types, namely:
It has been found that a large
number of monoterpenoid derivatives belonging to these categories invariably
occur naturally in the purest optically active form; however, certain plant
species do have both enantiomers, such as: Pinus species contain both
(+)- and (–)-α – pinene.
A few typical examples of
monopenoids found in naturally occurring plant species are described under: camphor,
eucalyptol, menthol and thymol.
________________________________________
*
Monomeric: An
entity or a unit from which polymer is formed.
2.1.1 Camphor
Synonyms Gum camphor; Japan camphor; Formosa camphor. Laurel camphor.
Biological Source It occurs in all parts of the camphor tree, Cinnamonum camphora.T. Nees & Ebermeier, belonging to family Lauraceae.
Geographical Source The word camphora is derived from the Arabic Kafur, meaning chalk. The camphor tree, which is a huge evergreen plant, is found to be indigenous to Japan, China and Taiwan. It has also been naturalized specifically in the Mediterranean region eg; Algeria, Tunsia, Libya, Egypt, Italy and Greece. Besides it is grown in South Africa, Ceylon, Brazil, Jamaica, Florida and California. History reveals that Borneo camphor (from Borneol) arrived in Arabia in the sixth century and in Europe in the twelth century. Earlier, the worlds 80% supply of natural camphor was provided by Taiwan (Formosa) alone and the rest 20% by Japan and Southern China. Soonafter the second World War (1945) the commercial production of synthetic camphor has more or less catered for the ever increasing demand of camphor in the world market.
Preparation It is prepared from the chipped wood by subjecting it to steam distillation and subsequently collecting the distillate in specifically designed chambers where camphor will solidify on its miner walls upon colling and may be collected later on from the bottom of the chamber. The crude solidified camphor is purified by mixing it with a suitable proportion of soda lime, sand and charcoal; and subjecting the mass to sublimation at controlled temperature when pure crystals of camphor would be collected as a sublimate. It is finally compressed into either small cubes or thin plates, wrapped and exported.
Camphor from Volatile Oils It may be prepared from volatile oils by two simple methods, namely:
Methods-I In case, the oil contains a substantially large proportion of camphor, it may be separated
by deep freezing or sudden chilling; and if the camphor content in oil is not so much it is mostly fractionated and the camphor containing fraction is chilled to recover camphor.
Method-II Camphor may be recovered from volatile oils by the instant production of insoluble complexes with strong mineral acids eg; sulphuric acid 80% (30N).
Synthetic Camphor (or Borneol Camphor) The camphor is obtained commercially from α-pinene present in the turpentine oil through several steps sequentially e.g., treatment with HCl, isomerization, treatment with KOH and finally oxidation with HNO3 as given below:
Colour : Translucent mass with crystalline fraction
Odour : Characteristic odour
Optical Activity : Natural camphor = Dextro rotatory (+ 41o to 43o) Synthetic camphor = Racemic mixture;
Solubility : Soluble in water (1:600)
Chemical Structure Camphor is a bycyclic terpenoid ketone as given below:
In the presence of platinum black it undergoes hydrogen at ambient temperature giving rise to isoborneol as the major product and traces of borneol.
Its prolonged hydrogenation often yields camphene.
2.1.2 Eucalyptol
Synonyms Cineole; Cajeputol.
Biological Source It is obtained from the leaves of Eucalyptyus globulus Labill, belonging to family Myrtaceae.
Geographical Source The eucalyptus tree is a native of Australia and Tasmania. It is largely cultivated in Calfornia, Spain, Portugal and India. In India it is abundantly found in the Himalayan region, Nilgiri district, Kumaon Hills and Assam.
Preparation A number of volatile oils from certain Eucalyptus species invariably contain eucalyptol as high as 30 to 70%. It also occurs in cajuput oil (40%) and in laurel leaf oil(50%). However, eucalyptol may be isolated from these oils by adopting one of the following methods:
Method 1 By subjecting the volatile oil to fractional distillation and collecting the fractions between 170-180oC to obatin crystals of cineole at –10oC (m.p. + 1.5oC)
Method-2 Cineole forms addition compounds with halogen acids, e.g., C10H18O HCl and C10H18O. HBr; with phosphoric acid as C10H18O.H3PO4 which also serve as a means of its purification, and
Method 3 Eucalyptol yields an addition product with a 50% (w/v) alcoholic solution eg; C10H18O. C6H6O2 (mp 82-85oC), from which the former may be generated.
Note: This method is mostly applicable to such volatile oils that have a higher cineole content.
Synthetic Method Eucalyptol may be prepared synthetically by the dehydration ofterpin hydrate as given below:
Description
Colour : Colurless or pale yellow liquid.
Odour : Camphoraceous and aromatic.
Taste : Pungent and leaves a cold sensation.
Solubility : Water insoluble; soluble in paraffin, fixed oils and ethanol 90%.
Chemical Structure
Eucalyptol is an epoxy or oxido derivative of p-menthane. and is also known as 1,8-epoxy-pmethane or 1,8-oxido-p-menthane. It is found to be quite stable and hence may be distilled over metallic sodium safely without undergoing any change whatsoever. It is not affected by the action of reducing agents.
Chemical Tests
1. When a drop of eucalyptol is carefully treated with a drop of 5% (w/v) solution of hydroquinone in alcohol on a slide, it forms either colourless prisms or rhomboids; but with a 50% (w/v) solution of resorcinol in alcohol leaf-like crystals are obtained.
2. It forms characteristic addition compounds with HCl, HBr and H3PO4 with well defined melting points.
Uses
1. It is used internally as a stimulating expectorant to relieve severe cough and in bronchitis in the form of inhalatious.
2. It is abundantly employed externally as a mild anaesthetic and antiseptic for the treatment of various inflammatory conditions.
2.1.3 Menthol
Synonyms 1-Menthol; 3-Menthanol; Menthan-3-ol; Peppermint camphor, Hexahydrothymol.
Biological Sources It is found in the peppermint oil obtained from the fresh flowering tops of the plants commonly known as Mentha piperita Linn., or other allied species ofMentha, belonging to family Labiatae.
Geographical Source Various mentha species are duly cultivated in various parts of the world. It grows both abundantly and widely in Europe, while it is cultivated in Japan , Great Britain, Italy,France, United States, CIS countries, Bulgaria and India.
Preparation It is normally prepared from Japanese Peppermint Oil, from the flowering tops of Mentha avensis Linne’ var piperascens, by subjecting it to refrigeration below –22oC whereby the menthol crystallizes out distinctly. The crystals of menthol are separated by filteration and squeezes between layers of filter papers to remove the adhering oil and finally purified by recrystallization.
Synthetic racemic menthol is prepared by the hydrogenation of either pulegone orthymol as shown below:
It may also be prepared from pinene.
Description
Colour : Colourless
Odour : Pleasant peppermint like odour
Taste : Characteristic, aromatic and cooling taste
Shape : Hexagonal cyrstals usually needle like, prisms; crystalline powder; fused masses.
Chemical Structure Menthol has three chiral centres (*), hence it would give rise to eight (23) optically active isomers and four racemic forms. Menthol on oxidation gives menthone (a ketone), by the sacrifice of one chiral centre; therefore, the resulting menthone must exist in four (22) optically active isomers and two recemic forms and all, these have been actually prepared.
Special Features following are the special features of menthol, namely:
(a) Dehydrogenation: Menthol first on dehydration yields two isomeric forms of p-menthane, which on subsequent dehydration gives rise to p-cymene as follows:
(b) Reduction: Menthol on reduction with hydroiodic acid yields p-menthane as under:
Chemical Tests
1. When 10 mg crystals menthol are first dissolved in 4 drops of concentrated sulphuric acid and then a few drops of vanillin sulphuric acid reagent are added it shows an orange yellow colouration that ultimately changes to violet on the addition of a few drops of water.
2. A few crystals of menthol are dissolved in glacial acetic acid and to this solution a mixture of 3
drops of H2SO4 and 1 drop of HNO3 are added. It fails to produce either green or bluish green colouration (Thymol gives a green colouration).
3. Menthol provides a plethora of compounds of diagnostic value for differential identification, for instance: menthoxy acetate; p-nitrobenzoate; d-camphor sulphonate; acid phthalate; phosphoric acid-complex; and 3,5-dinitrobenzoate.
Uses
1. It is used profusely in various types of mouth washes, toothpastes and similar oral formulations.
2. It finds its enormous use as a flavouring agent for chewing gums, candies, throat lozenges and also certain mentholated cigarettes.
2.1.4 Thymol
Synonyms Thyme camphor; m-Thymol; 3-p-Cymenol; 3-Hydroxy-p-cymene;
Biological Sources It is obtained from the essential oil of Thymus vulgaris L., (Thyme oil); Monarda punctata L., (Horsemint oil), and Monarda didyma L., (Oswego tea oil),belonging to family Lbiatae. It may also be derived from Carum capticum Bentham er Hooker, (Ajowan oil), belonging to family Umbelliferae, and several species of Ocimum,for instance: Ocimum gratissimum L. (Tulsi oil), belonging to family Labiatae.
Geographical Source T. vulgaris is grown and cultivated abundantly in many parts of Europe, Australia and North Asia.
Preparation Thymol may be extracted from thyme oil by agitation with dilute aqueous alkali solution (= 5% w/v in water). The aqueous layer is first separated and subsequently made acidic with dilute acid, when thymol gets separated as an oily layer floating on the surface that may be recovered either by extraction with ether or by steam distillation.
Another means of obtaining thymol from thyme oil is to subject the latter to very low temperature (–25oC) when thymol separates as crystals.
Synthetic Thymol The thymol of commerce may be prepared synthetically by anyone of the following routes, namely:
(a) From Menthone: Menthone is first treated with bromine. and then quinoline to produce thymol:
(b) From m-Cresol: m-Cresol on being treated with isopropanol in the presence of a suitable catalyst yields thymol.
(c) From Piperitone: When pipertone, usually obtained from the Australian Eucalyptus oils, is treated with ferric chloride it gives rise to thymol.
Description
Colour : Transparent, colourless
Odour : Aromatic thyme—like odour
Taste : Pungent taste
Solubility : In water (1: 1200); in alcohol (1:1), in glycerol (1: 1000); Freely soluble in ether, chloroform, carbon disulphide, benzene and glacial acetic acid; soluble in fixed oil and volatile oil.
Chemical Structure The phenolic OH moiety present in thymol enables it to form salts of acetate and carbonate easily which are used as antiseptic and anthelmintic respectively.
Thymol when disolved in NaOH solution and treated with an I2-KI solution it forms thymol iodide that finds its use as an anti-infective and antifungal agent.
Chemical Tests
1. Thymol when fused with phthalic anhydride develops bright violet red to intense red colouration, and on adding dilute alkali it gives an intense blue coluration due to the formation of thymolphthalein.
2. Thymol on being dissolved in concentrated sulphuric acid yields the corresponding thymesulphuric acid [C6H2(SO3H) (CH3). (C3H7).OH], which produces a distinct violet colour with ferric chloride solution.
3. An alcoholic solution of thymol on being treated with FeCl3 solution does not produce any colouration.
Note: Carvacrol on identical treatment gives a green colouration.
4. A small crystal of thymol is dissolved in 1 ml of glacial acetic acid and to this is added one drop of HNO3 and six drops of sulphuric acid, when it exhibits a deep bluish green colour.
5. Dissolve 0.1 g of thymol in 2 ml of NaOH solution (10% w/v) and heat in a water bath to produce either a clear colourless solution or a pale red solution, that ultimately turns darker in shade on keeping without the separation of oily drops. If the resulting solution is shaken with a few drops of chloroform it gives a violet colouration.
6. Thymol forms definite derivatives with various reagents e.g., napthylurethane derivative (m.p.160oC); phenylurethane derivative (106-107oC).
Uses
1. It is invariably employed as an antifungal and antibacterial agent.
2. It is a vital component in several analgesic and topical antiseptic formulatios in low concentrations ranging between 0.1 to 1% in personal health care products.
3. It is widely employed in preparation exclusively intended for mouthwashes, gargles, oral preparations and as a local anaesthetic in toothache.
Source:Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar
Source:Pharmacognosy And Pharmacobiotechnology By Ashutosh Kar




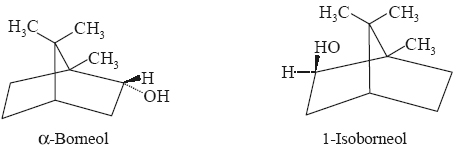

































0 Comment:
Post a Comment